 | Your browser does not display parts of our website correctly. We use the latest technology available to provide you with a high quality experience; please upgrade your browser to its latest version to view the contents properly: |
 Depomed, Inc. (DEPO) is a specialty pharmaceutical company. The firm develops products for pain and other conditions, and diseases of the central nervous system in the United States.
Depomed, Inc. (DEPO) is a specialty pharmaceutical company. The firm develops products for pain and other conditions, and diseases of the central nervous system in the United States.
It offers Gralise (gabapentin), an once-daily product for the management of postherpetic neuralgia; Zipsor (diclofenac potassium) liquid filled capsule, a non-steroidal anti-inflammatory drug for the treatment of mild to moderate acute pain in adults; CAMBIA (diclofenac potassium for oral solution), a non-steroidal anti-inflammatory drug indicated for acute treatment of migraine attacks in adults; and Lazanda (fentanyl) Nasal Spray, an intranasal fentanyl drug used to manage breakthrough pain in adults.
The company is also involved in the clinical development of DM-1992 that completed a Phase II trial for Parkinson's disease. Depomed, Inc. sells its Gralise products to wholesalers and retail pharmacies. It has license and collaboration agreements with Abbott Products Inc.; Santarus, Inc.; Boehringer Ingelheim International GMBH; Ironwood Pharmaceuticals, Inc.; Janssen Pharmaceutica N.V.; Janssen Pharmaceuticals, Inc.; Mallinckrodt; Patheon Puerto Rico, Inc.; and Valeant Pharmaceuticals International, Inc.
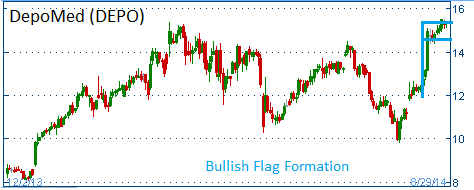
Shares have formed a bullish "flag" and higher share prices are expected for the stock.
52-Week Trading Range: $6.95 - $15.51
Entry Point: $15.25
Stop Loss: $14.48
Target Price: $16.78
Shares of DEPO are down 8% after the firm announced offering of $230 million of convertible notes. Shares typically take a bounce the day after convertible offerings. Closed at $14.10